

- #Rutgers periodic table chemistry how to#
- #Rutgers periodic table chemistry full#
- #Rutgers periodic table chemistry plus#
Below is a table relating the group numbers to the number of valence electrons.
#Rutgers periodic table chemistry full#
Meanwhile, group eighteen is the most stable as these elements have a full valence shell (eight valence electrons).
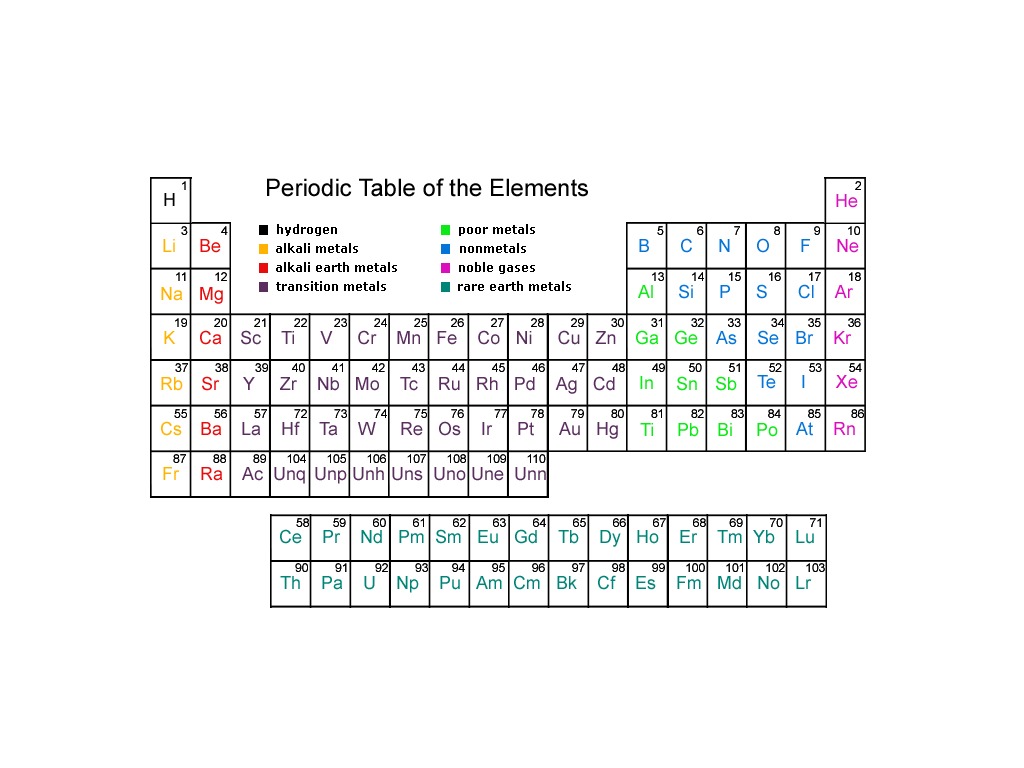
The first group is the least stable as it only has one valence electron. The first column on the left is group 1, and the last column on the right is group 18. There are 18 groups in the periodic table, one per each column of the periodic table. How many groups are in the periodic table? Moreover, the more filled the valence shell is, the more stable the element. These electrons are either donating, accepting, or sharing. The reason for this is that the valence electrons, which are the electrons in the outermost shell, are the ones taking part in chemical reactions. The number of valence electrons present dictates the properties of an element. There is eighteen groups on the periodic table in total, and each periodic table group contains elements with the same number of valence electrons. Period NumberĪs previously mentioned, the vertical columns on the periodic table are called “groups”. Below is a table to help visuals the periodic number and the corresponding orbitals. As you go down the rows, the number of orbitals increases. The top period, which contains hydrogen and helium, has only two orbitals. There are seven periods total and each element in a period has the same number of atomic orbitals. So what is a period on the periodic table? Periods are the horizontal rows of the periodic table.
#Rutgers periodic table chemistry plus#
There are 18 groups, and there are 7 periods plus the lanthanides and actinides. Groups are the columns of the periodic table, and periods are the rows. For a more in-depth explanation of periodic trends, click here. These trends tell you where the highest and lowest types of properties are concentrated on the periodic table. A quick way to understand an element’s chemical and physical properties is to know the periodic trends.
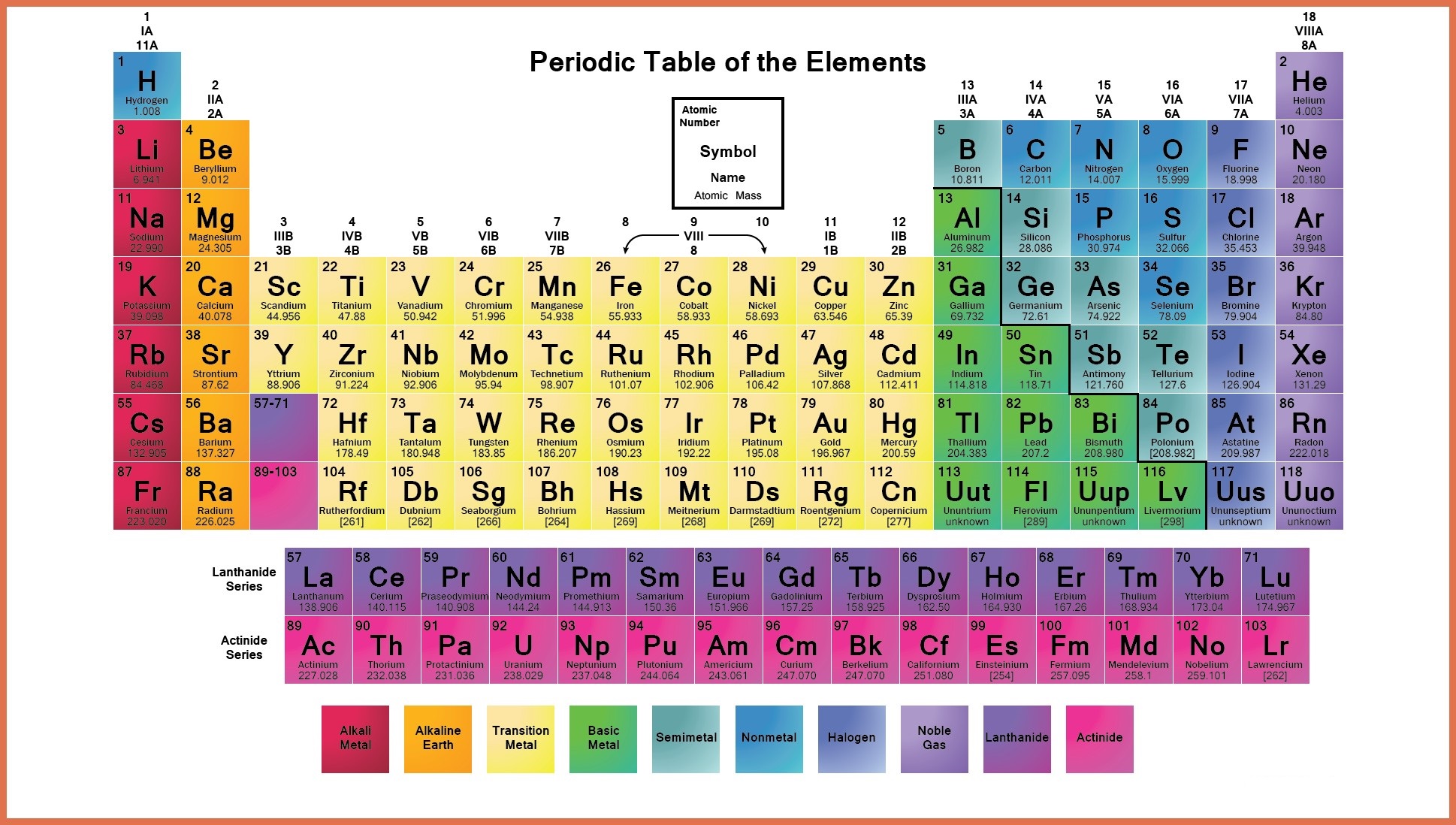
It is important to note how the location of elements on this table tells us about their properties. From this deduction, he formed the periodic table. In 1869 Russian chemist Dmitri Mendeleev noticed there existed an innate pattern of organization for the chemical elements. Meanwhile, elements in the same period have the same number of occupied electron shells. Elements in the same group have the same number of valence electrons. The periodic table is organized into groups (vertical columns), periods (horizontal rows), and families (groups of elements that are similar). The Periodic Table and the Periodic Trends We think our periodic table is one of the best in the world! Visit our interactive periodic table. Families: Elements that have the same number of valence electrons and therefore similar properties.Periods: The horizontal rows in the periodic table that signify the number of electron shells in an element.Groups: The vertical column of the periodic table that signifies the number of valence electrons in an element.Elements: A pure substance composed of a single atom.
#Rutgers periodic table chemistry how to#


 0 kommentar(er)
0 kommentar(er)
